Traction Maculopathy Vitreomacular Traction Syndrome, Cellophane Maculopathy, Macular Pucker, Macular Hole
As Published in Retinology Today in memoriam of Klaus Heimann, 1935-1999
Helen Keller Foundation for Research and Education
Robert Morris, M.D.
C. Douglas Witherspoon, MD
Ferenc Kuhn, MD, Ph.D.
Suzanne Nelson, R.N.
Brad Priester, MD
Richard Mayne, Ph.D.
See below for Physician’s Resources, Figures and References.
I. The Problem
Macular traction can be anterior/posterior (AP) as in vitreomacular traction syndrome. More commonly, traction is tangential to the macular surface in cellophane maculopathy, macular pucker, and macular hole. Traction on macular tissue produces gradual anatomic and functional deterioration in proportion to traction forces and their duration of action.
Vascular incompetence secondary to traction can occur as reflected in the abnormal fluorescein angiogram of certain epimacular proliferation (EMP) cases. Such vascular leakage can usually be reversed by removing epimacular proliferation, as is dramatically shown in a normalized postoperative angiogram. More subtlely, it is now commonly felt that even minimal AP or tangential traction can contribute to diffuse macula edema in diabetic eyes whose vessels are already abnormally permeable. Hutton first described restoration of vascular competency in such eyes with reduced fluorescein leakage and visual acuity improvement in certain cases by traction release alone.
Probably as a reflection of chronic (traction related) vascular incompetence, macular tissue may become diffusely edematous, even leading to the appearance of macular detachment on occasion. The fovea commonly develops cystic changes over time which are usually, but not always, reversible. In extreme cases, foveal cysts can develop into partial or full thickness holes if left untreated.
The generally accepted explanation for macular hole development in the aging eye is the theory of Gass which describes a foveal micro hole enlarging as a result of subtle centripetal traction forces (1). Accordingly, most vitreoretinal surgeons are now using surface traction release to increase the rate of successful macular hole closure.
Taken as a whole, vitreomacular traction syndrome, cellophane maculopathy, epimacular proliferation, and macular hole are estimated to occur in 6.4% of the U.S. population over age 50 (2). Thus, as many as 2.5 million persons in the US alone to some degree exhibit a form of age-related macular degeneration secondary to traction (traction ARMD). In contrast to the primary form of ARMD occurring subretinally, traction ARMD first affects the retinal surface and inner retinal layers, and is largely reversible if surgically addressed prior to the development of chronic secondary changes.
While traction maculopathy may first be noted simply as distorted vision, it can gradually progress to reduce best corrected visual acuity to 20/200 (0.1, legal blindness). Traction maculopathy can occur bilaterally in an estimated 10 – 30% of cases.
II. The Solution
A. Vitrectomy and Epimacular proliferation
Robert Machemer developed pars plana vitrectomy in 1970 and the procedure was gradually incorporated into patient care over the next few years. In 1976, Machemer first removed idiopathic EMP during vitrectomy using a bent-tipped, 22 gauge sharp needle (3). Just as in vitrectomy itself, epimacular surgery was initially reserved for only advanced cases in which visual acuity had deteriorated to the 20/70 – 20/200 level and a substantial membrane was evident. Under these circumstances a useful estimate was that a patient could expect to regain, on average, half of the lines of visual acuity lost from the effects of traction maculopathy (4).
Throughout the 1980’s, removal of epimacular proliferation became more common, and macular surgery began to constitute an ever larger percentage of vitrectomies performed. The daunting challenge of restoring foveal function by traction release became a necessary part of each vitreoretinal surgeon’s repertoire.
The EMP procedure is time limited due to the reported complication of photic maculopathy. It has been described by one excellent vitreoretinal surgeon as “nerve racking.” In fact, it requires the most delicate hand movements of any surgery on the human body. Analysis of EMP specimens in one report suggested that if long internal limiting membrane (ILM) fragments were present, visual return was reduced (5). Other analysis did not come to this conclusion (6). While membrane recurrence is uncommon, residual “cellophaning” frequently limits outcome. In addition to pics, evermore elegant forceps are now utilized for EMP removal.
B. Macular hole – a new understanding of the vitreoretinal interface
In 1988 Gass first put forth his theory that idiopathic macular hole resulted from expansion of a micro dehiscence at the fovea by tangential forces (1).
In 1991 Kelly and Wendel (7) first reported successful closure of macular hole with substantial return of visual acuity. The pace of progress in our understanding of both the vitreoretinal interface and of traction maculopathy and its treatment quickened significantly, as innovative surgeons endeavored to improve upon the author’s reported 50% anatomical hole closure rate. Appreciated for the first time was the role of even minimal surface traction that previously had been considered inconsequential in traditional surgery for epimacular proliferation. The importance of cortical vitreous removal and the significance of the Weiss ring as a proof of posterior vitreous detachment were also better understood.
C. The Internal Limiting Membrane (ILM) – It’s role in Traction Maculopathy
Coincidental with Kelly and Wendel’s report of macular hole closure, Morris, Kuhn, and Witherspoon in 1990 reported an eight year study of what we termed “hemorrhagic macular cysts (HMC) in Terson’s Syndrome (8). We showed that removal of the entire macular ILM by blood was followed by long term, stable and excellent visual acuity without substantial surface proliferation or “wound healing” response, after the loose ILM and blood were removed by vitrectomy. By 1993 we had begun using a procedure we called “ILM Maculorhexis” in the treatment of macular hole and epimacular proliferation (9). In 1994, we further predicted that removal of the internal limiting membrane might become an important part of surgery for all forms of traction maculopathy (10), (11).
Subsequent advocates of ILM removal such as Logan Brooks, Tom Rice, Tony Capone, Robert Wendel, and Claus Eckhardt, reported higher rates of macular hole closure with good visual results. Because the removal of ILM was technically difficult and prolonged macular surgery, and because of the possibility that it might affect the underlying neurosensory retina, ILM removal remained controversial. The use of pharmacological adjuvants to aid macular hole closure received hopeful attention, but did not prove to be substantially efficacious. By 1999, less than 20% of vitreoretinal surgeons continued to use adjuvants (12).
In 1997 Morris and Witherspoon designed the first “ILM forceps” (Grieshaber) that, for the first time, moved the instrument shaft outside the surgeon’s macular view by using a horizontal arm, gradually approaching the retina at a fine tip . This delicate forcep, when properly maintained and inspected prior to each use, allows direct ILM viewing and grasping without preliminary cutting or scraping. In a series of 94 consecutive vitrectomies for macular hole with ILM removal, 100% of our holes closed with visual return similar to other reported series of successful hole closures (13). It is our feeling that the ILM itself, together with any proliferation on its surface, contributes to hole stiffness, and that ILM removal in stage III and IV holes significantly increases the probability of macular hole closure with imperceptible edges.
As recently as 1998, there has been understandable skepticism among even experienced vitreoretinal surgeons that the tissue that we and others have been removing was truly ILM. However, by the October 1999 American Academy of Ophthalmology meeting in Orlando, this controversy was no longer evident. ILM can be reliably distinguished intraoperatively by its characteristic curvelinear disinsertion line during removal from the retina by forceps. Illuminated ILM fragments in the forceps after elevation from the retina also exhibit unique characteristics. The tissue is clear, with sharp edges and “scrolls” spontaneously. Confirmatory phase contrast microscopy postoperatively is reliable, quick and inexpensive.
In a series of 44 consecutive ILM microscopic specimens submitted by Morris and Witherspoon after macular hole surgery, ILM was confirmed by phase contrast microscopy (and randomly by transmission electron microscopy and type IV immunofluorescent antibody staining) as the primary tissue of each specimen. Neither substantial Mueller cell membrane fragments nor axonal fragments were seen underlying the removed ILM (13). Thus, we believe that the experienced surgeon can successfully identify and remove the ILM. Nevertheless, atraumatic mechanical removal of the ILM is difficult. In a poll of vitreoretinal surgeons conducted by the Vitreous Society in 1999, the majority of vitreoretinal surgeons attempt to remove the ILM in macular hole surgery, and 85% of respondents describe this procedure as difficult or very difficult (12).
Even without extensive surgical experience, reliable intraoperative identification of the ILM is now possible using the indocyanine green (ICG) ILM staining technique as described by Kim and Clark in 1999 (14). The ILM direct-view forceps (Grieshaber), combined with ICG staining, now make ILM removal much more predictable and rapid. Even as mechanical ILM removal has become easier, however, a better method is on the horizon.
D. Fluidic Internal Limiting Membrane Separation – FILMS
At the Vitreous Society in July 1998, and subsequently at the Retina Society and American Academy of Ophthalmology meetings of 1998, we introduced a new technique, Fluidic Internal Limiting Membrane Separation (FILMS), for predictable, rapid, and atraumatic removal of the ILM and all overlying proliferation (15). In the FILMS procedure, the FILMS cannula™ is inserted under the ILM in the peripheral macula. Using foot pedal control of a viscous fluid injector, viscoelastic fluid is slowly injected, establishing a cleavage plane between the ILM and the remaining neurosensory retina. Just as in the HMC’s of Terson’s syndrome, the FILMS cyst develops, but at a rate controlled by the surgeon. No petechial hemorrhages were seen, probably reflecting the fact that there is no mechanical pulling on the retina, but rather gentle tamponade of the retina, as the ILM/EMP complex is elevated. The separated tissue was then easily removed with forceps.
We removed the entire macular ILM and overlying proliferation in 6 cases of macular hole and 5 cases of cellophane maculopathy/macular pucker. All holes closed, and all EMP was removed without complication. Visual return was similar to that reported in well performed mechanical ILM/EMP removal with macular hole patients gaining an average of four lines of Snellen visual acuity improvement by one year post-operatively, and with non-chronic EMP cases achieving an average of five lines of visual improvement.
Instead of attempting to remove proliferation from the retinal surface, FILMS removes the abnormal retinal surface itself. Thus, FILMS is the first surgery performed within the retina, separating the neurosensory layers from all overlying pathologic tissue. Just as in Terson’s HMC’s, we have not seen substantial recurrent proliferation, apparently because cells have relatively greater difficulty adhering to the macular surface denuded of it’s ILM.
E. The ILM Denuded Macula
Intentional ILM removal has now been performed in thousands of eyes over the last five years by numerous surgeons, without apparent visual complications. Just as we have seen in follow up of Terson’s patients, the ILM denuded macula functions well long term. Why?
Mueller cell endfeet join together to line virtually the entire inner surface of the retina underlying the ILM. The contiguous Mueller cell membranes do not constitute a part of the ILM, but rather they are relatively loosely in contact with the overlying ILM. In a series of pig eyes, our colleague Clyde Guidry, Ph.D., removed the ILM by directly grasping and pulling on the gel vitreous in equatorially transected pig eyes. The ILM denuded retina was then subjected to gentle chemical digest. Mueller cells were routinely harvested and confirmed to have intact cell membranes by phase contrast microscopy. These cells were then successfully grown in culture, reflecting their viability (16). Thus, we believe that the ILM denuded macula in both Terson’s Syndrome and in the surgically treated human macula has an intact surface of confluent Mueller cell membranes. Using the FILMS microcannula we hope to demonstrate this histologically in the primate eye.
In addition to our long term study of Terson patients after loss of the ILM, we have now performed multi-focal ERG on one eye, fourteen months after ILM/EMP removal by the FILMS technique. The multi-focal ERG was normal, and visual acuity had recovered to 20/20 (1.0), from 20/50 (0.4) pre-operatively.
III. The Future
FILMS* makes possible a rapid, atraumatic removal of all surface traction in all types of traction maculopathy. Because it involves only one critical maneuver (FILMS cannula™ placement) instead of many, it is substantially easier to perform than traditional mechanical techniques and inherently safer. Thus, all forms of traction maculopathy are potentially curable by the FILMS method before substantial deterioration of the macula has occurred.
*The FILMS microcannula™ is patented, US patent #6210357 and the FILMS surgical method is #6024719. Some of the authors have a potential financial interest in this product.
All authors are affiliated with the Helen Keller Foundation for Research and Education Birmingham, Alabama, USA. Drs Morris, Witherspoon and Kuhn are faculty members in the University of Alabama at Birmingham Department of Ophthalmology. Drs. Morris (1976-77) and Kuhn (1984) are former fellows of Dr. Heimann. Dr. Mayne is Vice Chairman of the UAB Department of Cell Biology. This work has been partially sponsored by the Helen Keller Eye Research Foundation.
Physician’s Resources:
As Published in Retinology Today
Figures:
 |
 |
 |
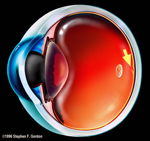
| FIGURE 1 THE HUMAN EYE WITH MACULAR CENTER OF VISION MARKED BY ARROW ADJACENT TO THE OPTIC NERVE. |
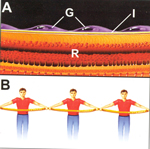
FIGURE 2 (A) ABNORMAL GLIAL CELL MEMBRANES (G) ATTACHED TO THE MACULAR SURFACE (I = ILM) BEFORE CONTRACTION (R = RETINA). ANALOGOUS TO A LINE OF MEN HOLDING A ROPE (B). |
FIGURE 3 ( C) EMP AFTER CONTRACTION TO PRODUCE MACULAR PUCKER. ANALOGOUS TO A LINE OF MEN SHORTENING A ROPE (D). |
 |
 |
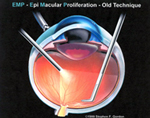
| FIGURES 4 & 5 CURRENT FORCEPS TECHNIQUE OF REMOVING EMP / ILM BY RECURRENT GRASPING AND PEELING. |
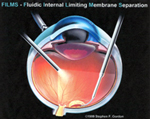
FIGURES 6 & 7 FILMS CANNULA™ INSERTED UNDER SCARRED RETINAL SURFACE LAYER. GRADUAL INJECTION OF CLEAR, VISCOUS FLUID SEPARATES EMP / ILM COMPLEX FOR SUBSEQUENT EASY REMOVAL FROM THE EYE BY FORCEPS. |
References: